Migrated intrauterine device (IUD) - late uterine perforation during lactation
Image Description
Uterine perforation post IUD insertion is rare and may occur either as immediate uterine perforation - caused by a traumatic insertion, or, late uterine perforation - caused by the progressive erosion through the myometrium. Current professional recommendations give no eligibility restriction for postpartum IUD insertion, regardless of whether or not the patient is breast-feeding.
We present the case of a 34-year-old patient, who had an IUD (T-Cu 380A, 32 mm) inserted at 12 weeks postpartum, on an anteverted uterus, without any incidents during the insertion. Approximately four months after the insertion, the patient was admitted for mild, intermittent pelvic pain, light bloody vaginal discharge, and digestive complaints. During the gynaecological consult, no withdrawal strings were seen in the cervix. The transvaginal ultrasound confirmed the absence of the device from the uterine cavity. It could not be seen in the myometrium, in the peritoneal cavity, or in the bladder.
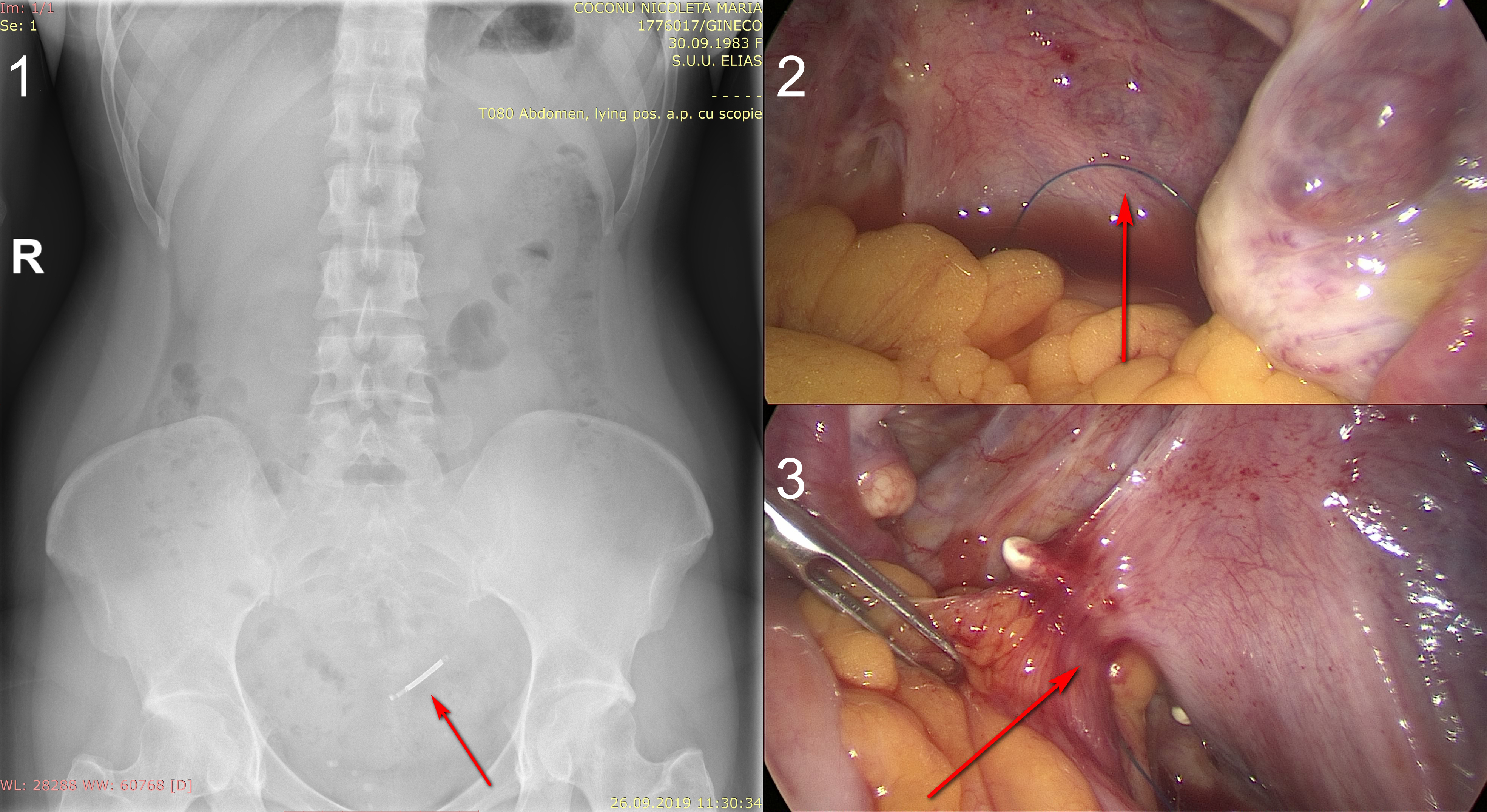
The abdominal X-ray (Figure 1) identified the parauterine location of the device. The laparoscopic removal of the migrated intrauterine device was performed. The device was found in the posterior uterine wall, partially penetrating it, in an uncommon position if we are to consider the literature. It had a transversal intra-myometrium arm - a D2 type perforation, apparently – but the longitudinal axis and the withdrawal strings were in the peritoneal cavity (Figure 2), being loculated by the epiploon and intestinal loops (Figure 3), with lax adherences, which enabled the device extraction rather easily, without any contiguity lesions.
References
Rowlands, S., Oloto, E., &Horwell, D. H. (2016). Intrauterine devices and risk of uterine perforation: current perspectives. Open access journal of contraception, 7, 19–32. doi:10.2147/OAJC.S85546
Intrauterine contraception: uterine perforation – updated information on risk factors Drug Safety Update June 2015. London, UK: Medicines and Healthcare Products Regulatory Agency; 2015.Available rom: https://www.gov.uk/government/uploads/system/uploads/attachment_data/ file/438800/Drug_Safety_Update_-_June_2015_pdf.pdf